TECHNICAL PAPER # 35
UNDERSTANDING EVAPORATIVE
COOLING
By
Eric Rusten
Technical Reviewers
Michael Bilecky
Dr. Agustin F. Venero
Published By
VITA
1600 Wilson Boulevard, Suite 500
Arlington, Virginia 22209 USA
Tel:
703/276-1800 . Fax: 703/243-1865
Internet: pr-info@vita.org
Understanding Evaporative Cooling
ISBN: 0-86619-246-8
[C]1985,
Volunteers in Technical Assistance
PREFACE
This paper is one of a series published by Volunteers in
Technical
Assistance to provide an introduction to specific
state-of-the-art
technologies of interest to people in developing countries.
The papers are intended to be used as guidelines to help
people choose technologies that are suitable to their
situations.
They are not intended to provide construction or
implementation
details. People are
urged to contact VITA or a similar organization
for further information and technical assistance if they
find that a particular technology seems to meet their needs.
The papers in the series were written, reviewed, and
illustrated
almost entirely by VITA Volunteer technical experts on a
purely
voluntary basis.
Some 500 volunteers were involved in the production
of the first 100 titles issued, contributing approximately
5,000 hours of their time.
VITA staff included Maria Giannuzzi
as editor, Suzanne Brooks handling typesetting and layout,
and
Margaret Crouch as project manager.
The author of this paper, VITA Volunteer Eric Rusten,
specializes
in technology and international development, and has worked
in
Kenya and Nepal. The
reviewers are also VITA volunteers.
Michael
Bilecky is partner and president of von Otto and Bilecky, an
engineering, construction, and energy management firm
located in
Washington, D.C. Agustin Venero specializes in research and
development in new energy sources for the OMICRON Technology
Corporation in Berkeley Heights, New Jersey.
VITA is a private, nonprofit organization that supports
people
working on technical problems in developing countries.
VITA offers
information and assistance aimed at helping individuals and
groups to select and implement technologies appropriate to
their
situations. VITA
maintains an international Inquiry Service, a
specialized documentation center, and a computerized roster
of
volunteer technical consultants; manages long-term field
projects;
and publishes a variety of technical manuals and papers.
UNDERSTANDING EVAPORATIVE COOLING
by VITA Volunteer Eric Rusten
I. INTRODUCTION
Cooling through the evaporation of water is an ancient and
effective
method of lowering temperature.
Both plants and animals
use this method to lower their temperatures.
Trees, through the
process of evapotranspiration, for example, remain cooler
than
their environment.
People accomplish the same thing when they
perspire. For both
trees and people the underlying scientific
principle is the same: when water evaporates, that is,
changes
from a liquid to a gas, it takes heat energy from the
surrounding
environment, thus leaving its environment cooler.
We have all experienced the result of evaporative
cooling. Sitting
under a tree on a hot afternoon is much cooler than sitting
either in the direct rays of the sun or in the shade of a
building.
As water from the tree's leaves evaporates, the air
surrounding
the tree is gently cooled.
Moreover, we have all felt
the cooling effect of perspiration evaporating from our
skin.
Finally, some of us may have discovered that water kept in a
canvas bag, porous clay container, or in a canteen with a
water-soaked
cloth cover, is much cooler, especially on a hot day, than
water kept in plain metal or plastic containers.
As the water
evaporates from the surfaces of these containers it draws
heat
away from the containers and the water they hold, as well as
from
the air around them, thus leaving the water cooler.
Since it is possible to cool trees, water bottles, and
ourselves
by this process shouldn't it be possible to cool other
things,
such as food and dwellings?
The answer to this question is a
definite yes.
Several systems have been designed to use the
principle of evaporative cooling to keep homes cool and
comfortable.
Also, methods have been developed that reduce the
temperature
of foods, such as fruits, vegetables, and dairy products,
far enough to retard spoilage.
Although lowering the temperature of fruits and vegetables
to
levels that retard spoilage is an important benefit of
evaporative
cooling, it is not the only one.
Evaporation not only
lowers the air temperature surrounding the produce, it also
increases the moisture content of the air.
This helps prevent
the drying out of produce, and therefore extends its
shelflife.
In general, evaporative cooling can be used where:
1.
temperatures are high;
2.
humidity is low;
3.
water can be spared for this use; and
4.
air movement is available (from wind or
electric fans).
This paper provides an introduction to the process of
evaporative
cooling. In
addition, the natural limitations and problems associated
with this process, along with some practical applications
of evaporative cooling are examined.
II. BASIC PRINCIPLES OF EVAPORATION AND EVAPORATIVE COOLING
As noted earlier, evaporation is the process of changing a
liquid
into a gas. In this
case liquid water becomes water vapor, and
this gas becomes part of the mixture of gases that compose
the
air. The change from
the liquid state to a vapor requires the
addition of energy, or heat.
The energy that is added to water to
change it to a vapor comes from the environment, thus
leaving the
environment cooler.
Not all substances need to gain or lose the same amount of
energy
to change from one physical state to another.
For example, it
takes much more heat energy to cause a given amount of water
to
vaporize than to cause the same amount of alcohol to do so.
Water is unique in that it requires a relatively large
quantity
of heat energy to change from a liquid to a gas.
It is this
characteristic that enables evaporating water to lower
substantially
the temperature of its environment.
On the other hand, the amount of water vapor that can be
taken up
and held by the air is not constant; it depends on two
factors.
The first is the temperature (energy level) of the air,
which
determines the potential of the air to take up and hold
water
vapor. The second
factor is the availability of water. If
little
or no water is present, the air will be unable to take up
very
much.
The measurement of the amount of water vapor present in the
air
is spoken of as the air's humidity.
There are two ways of
measuring the humidity of the air: (1) absolute humidity and
(2)
relative humidity.
Absolute humidity is the measurement of the
actual quantity of water (measured in grams) in a given
volume of
air (measured in cubic meters or liters).
Relative humidity, the
more common measurement, is the measurement of the water
vapor in
the air as a percentage of the maximum quantity of water
vapor
that the air would be capable of holding at a specific
temperature.
Air that is fully saturated--that is, contains as much
water vapor as possible--has a relative humidity of 100
percent,
while air that has only half as much water vapor as it
possibly
could hold at a specific temperature has a relative humidity
of
50 percent.
The relative humidity varies with the temperature.
As the air
cools (i.e., loses energy), its ability to hold water vapor
decreases, which results in an increase in the relative
humidity.
This is because the ability of the air to hold water vapor
has
been reduced by the drop in temperature, but the absolute
humidity
(the actual amount of water vapor in the air) has remainde
unchanged. If the
air temperature continues to fall the relative
humidity will approach 100 percent, or complete saturation.
The point at which the air is fully saturated is referred to
as
the dew point. At
temperatures lower than the dew point, water
vapor condenses out of the air onto cooler surfaces.
DETERMINING RELATIVE HUMIDITY
Before attempting to implement any of the evaporative
cooling
systems discussed in Section III of this paper, it is
necessary
to determine if environmental conditions, particularly the
relative
humidity, are suitable for the evaporative cooling process.
In some situations it may be possible to use already
existing
data, but where this information is not available it will be
necessary to collect it.
The following materials are needed to determine relative
humidity:
a thermometer, a small piece of cloth, a small glass or
plastic vial for water, and two pieces of cardboard or some
other
stiff material (the pieces of cardboard should be longer
than the
thermometer and as wide as half its length).
The procedure to determine relative humidity involves two
steps.
First, use the thermometer to determine the temperature of
the
air; note this down as the dry-bulb temperature (i.e., the
temperature
taken with the bulb of the thermometer kept dry).
Second, secure a small piece of cloth to the bulb of the
thermometer with some thread.
The end of the cloth should extend
beyond the tip of the bulb.
Then attach the thermometer to the
piece of cardboard.
Next, attach the small plastic or glass vial
to the cardboard just below the end of the thermometer so
that
the piece of cloth will fit in the vial.
The cloth covered bulb
of the thermometer should be left exposed to the air.
Figure 1
uecfg1x5.gif (540x540)

shows the final set-up of this apparatus.
Now, fill the vial with water so that the cloth and the bulb
will
be kept wet. Using
the other piece of cardboard, fan the lower
end of the apparatus for 30 to 60 seconds.
At the end of this
time note down this temperature as the wet-bulb temperature
(i.e., the wet-bulb thermometer temperature taken with the
bulb
end of the thermometer kept wet).
Repeat the final steps
several more times to ensure accuracy.
Add all of the wet-bulb
temperatures together and calculate the average wet-bulb
temperature.
Use the dry- and wet-bulb temperatures, and the charts in
Appendix A
uec1a400.gif (600x600)

determine the relative humidity for more than one time of
the
day, and for more than one day.
Several calculations over the
middle portions of a day, several times a month should be
enough
to determine if evaporative cooling would be effective in a
specific environment.
Exactly how relative humidity data are
used to determine the effectiveness of evaporative cooling
will
be discussed later.
FACTORS AFFECTING EVAPORATION
As discussed earlier, evaporation results in cooling of the
air
or other substances.
As the rate of evaporation increases so
does the rate of cooling.
To make the most effective use of this
technology it is important to understand the factors that
influence
the rate of evaporation, and the relationships that exist
between these factors.
There are four major factors that affect the rate of
evaporation.
Although each of these factors will be discussed
independently,
it is important to keep in mind that they usually interact
with
each other to influence the overall rate of evaporation, and
therefore the rate and extent of cooling.
Factor 1: Relative Humidity
Relative humidity, as mentioned earlier, is the measurement
of
the amount of water vapor in the air as a percentage of
the maximum quantity that the air is capable of holding at a
specific temperature.
When the relative humidity is low, only a
small portion of the total possible quantity of water vapor
that
the air is capable of holding, is being held.
Under this situation
the air is capable of taking on additional moisture, and if
other conditions are also met, the rate of evaporation will
be
higher. On the other
hand, when the relative humidity is high,
the rate at which water evaporates will be low, and
therefore
less cooling will occur.
Under such conditions of high relative
humidity, evaporative cooling may not be effective.
However, in
many areas with high relative humidity, such as the humid
tropics, evaporative cooling can be effective if a dessicant
(e.g., silica gel) is used to remove moisture from the air
before it is cooled.
Factor 2: Air Temperatures
Evaporation, as stated earlier, occurs when water absorbs
sufficient
energy to change from a liquid to a gas.
Air with a
relatively high temperature will be able to stimulate the
evaporative
process and also be capable of holding a relatively
great quantity of water vapor.
Therefore, areas with high temperatures
will have higher rates of evaporation, and more cooling
will occur. With
lower air temperatures, less water vapor can be
held, and less evaporation, and cooling will take place.
Factor 3: Air Movement
Air movement, either natural (i.e., wind) or manmade (i.e.,
with
a fan), is an important factor that influences the rate of
evaporation.
As water evaporates from a surface it tends to raise
the humidity of the air that is closest to the water's
surface.
If this humid air remains in place, the rate of evaporation
will
start to slow down as humidity rises.
On the other hand, if the
humid air near the water's surface is constantly being moved
away
and replaced with drier air, the rate of evaporation will
either
remain constant or increase.
Factor 4: Surface Area
The area of the evaporating surface is another important
factor
that affects the rate of evaporation.
The greater the surface
area from which water can evaporate, the greater the rate of
evaporation. A
simple example will demonstrate the importance of
surface area to evaporation.
Consider the following two situations.
(1) one liter of water placed in a narrow glass container
with only about 16 [cm.sup.2] of surface area exposed to the
air;
and (2) another liter of water poured into a large shallow
pan with about 180 [Cm.sup.2] of surface exposed to the
air. Of
these two situations, which one could be expected to dry up
first, if both where left under the same environmental
conditions?
Because of the large surface area, the large pan of water
would dry up much sooner than the jar.
Even though each of these factors has its own separate and
significant
effect on the rate of evaporation, when combined, their
impact is much greater.
For example, the first two factors can
be discussed together in terms of wet- and dry-bulb
temperatures.
Under conditions where the difference between the wet- and
dry-bulb
temperatures is great, the rate of evaporation will also be
great. The graph in
Figure 2 should help explain this situation.
uecfg2x8.gif (600x600)

Curve A traces the change in the air temperature (dry-bulb
temperature)
over a 24-hour period; Curve B traces the wet-bulb
temperature, also recorded over a 24-hour period.
The difference
between the wet- and dry-bulb temperatures is the greatest
during
the period from 10:00 a.m. to 8:00 p.m.
From this it can be
reasoned that the relative humidity over this period was
low.
This is also the time period with the highest average air
temperatures.
Thus, under these conditions it can be assumed that
the rate of evaporation would be relatively great.
If the two
other factors, air movement and surface area, are applied
effectively,
the rate of evaporation would show an additional
increase.
MAXIMUM COOLING POTENTIAL
The extent to which evaporation can lower the temperature of
a
container or the air depends upon the difference between the
wet- and
dry-bulb temperatures.
Theoretically, it is possible to
bring about a change in temperature equal to the difference
in
these two temperatures.
For example, if the dry- and wet-bulb
temperature were 35[degrees]C and 15[degrees]C respectively,
the maximum drop in
temperature due to evaporative cooling would theoretically
be
20[degrees]C. In
reality, though, while it is not possible to achieve
100 percent of the theoretical maximum temperature drop,
however,
a substantial reduction in temperature is possible.
Depending on the environmental conditions, and the method of
evaporative cooling used, it should be possible to achieve
between
50 and 80 percent of the theoretical maximum drop in
temperature.
In the example given above, this would have resulted
in a temperature reduction of between 10 and 16[degrees]C.
III. DESIGN VARIATIONS
There are two general methods of evaporative cooling: direct
and
indirect. Direct
evaporative cooling involves the movement of
air past or through a moist material where evaporation, and
therefore cooling, occurs.
This cool moist air is then allowed
to move directly to where it is needed.
In contrast to this
process, indirect evaporative cooling uses some form of heat
exchanger that uses the cool moist air, produced through
evaporative
cooling, to lower the temperature of drier air.
This cool
dry air is then used to cool the environment, and the cool
moist
air is expelled.
In situations where cool dry air is more desirable than cool
moist air, the extra effort or expense involved in building
or
buying and using a heat exchanger may be justified.
On the other hand,
many situations exist where it will be better to use the
less complex and less costly direct evaporative cooling
process.
Evaporative cooling technology is used to cool rooms, homes,
food, or water. The
method of evaporative cooling used, direct
or indirect, depends on: (1) the specific needs of the
environment
that will be cooled; (2) the availability and cost of
commercial
energy; and (3) the amount of money and skill available
to buy or build the cooler.
The following discussion will present specific examples of
how
both methods of evaporative cooling can be applied.
The advantages,
disadvantages, and limitations of each of these applications
are also examined.
DIRECT EVAPORATIVE COOLING
One of the simplest and most commonly used forms of
evaporative
cooling is used to cool water.
This system usually uses either a
porous clay container or a watertight canvas bag in which
water
is stored. These
containers are then either hung or placed so
that the wind will blow past them.
The water in the containers
slowly leaks through the clay or canvas material and
evaporates
from the surface as warm dry air flows past.
This process of
evaporation slowly cools the water.
Small bottles, bags, or jars of produce, medicine, or dairy
products can be suspended in the water so they can be kept
cool.
This method of evaporative cooling is common among street
vendors
of South Asia, who use it to cool soda pop and fruit for
their
customers.
This type of evaporative cooler has limited
application. One of
the primary limitations is that the drop in temperature will
generally be only a small fraction of the total temperature
reduction that is possible.
This is primarily due to the large
volume of water that needs to be cooled by a relatively
small
evaporating surface area.
Secondly, only a small number of items
can be placed in large water containers.
The following section of
this paper outlines some common examples of other
evaporative
coolers. Before any
of these types of coolers are built or installed,
it is necessary to consider the probable effectiveness
of evaporative cooling in the specific environment and to
balance
the benefits gained against costs incurred.
The following section of this paper outlines some common
examples
of other evaporative coolers.
Outdoor Curtain Cooler
A variation of the simple process described above can be used
to
cool small outdoor areas (Figure 3).
In its simplest form this
uecf3x11.gif (540x540)
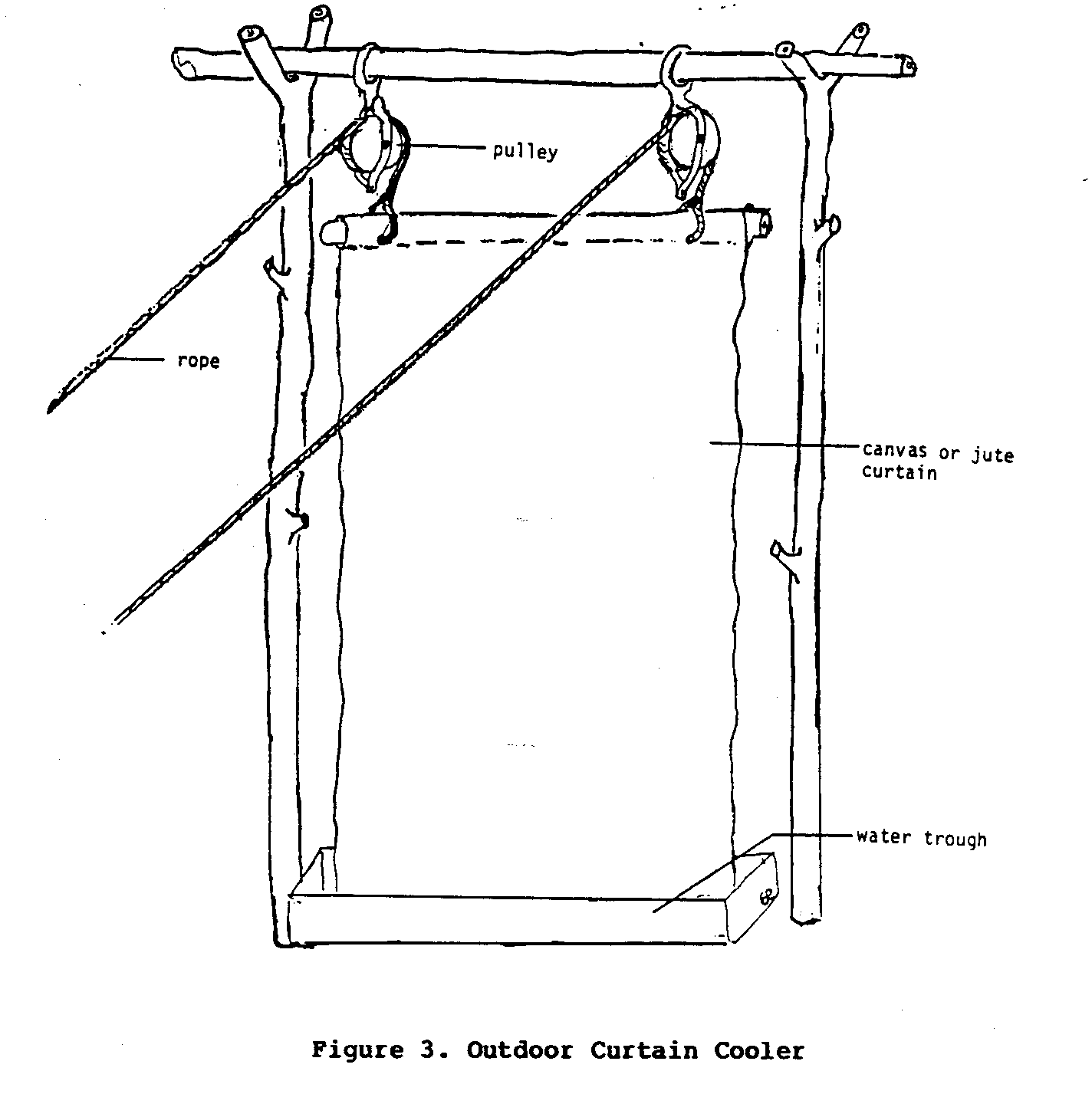
involves the use of a sheet of canvas or some other strong,
absorbent cloth as an evaporating surface.
The upper edge of the
canvas sheet is suspended by ropes that are usually held up
by
pulleys so that the sheet can be lowered and raised
easily. The
lower end of the sheet is secured in a trough of water large
enough to permit all of the sheet to fit.
When a cooler environment
is desired the canvas sheet is lowered into the trough of
water so that it becomes soaked with water, after which, it
is
raised. As hot, and
generally dry, air passes through and around
the moist cloth, evaporation occurs, which in turn cools the
air.
This cool moist air then cools the immediate environment.
Obviously, the size of the area that can be cooled using
this
method is limited.
Moreover, this cooler can not substantially
lower the air temperature.
Even with these shortcomings, people
who have used these simple coolers have said that they do a
fairly effective job of making the immediate environment
more
comfortable. The
simple nature of this cooler is its primary
advantage. If a more
comfortable outside environment is desired,
but cost is an important consideration, this cooler may be a
good
choice.
Indoor Curtain Cooler
Therather simple device described above can be adapted for
use
indoors. Again,
canvas, jute cloth, a coconut husk mat, or some
other absorbent material is used to expose water to moving
air.
For use indoors, such a cooling device requires some form of
energy source, generally electricity, to power a fan to blow
the
air through the absorbent material.
A small water pump is also
needed to circulate water from a lower trough to an upper
one.
This keeps water continually flowing through the absorbent
material so evaporation can occur.
Coolers of this type are used
extensively in the hot, dry areas of the western United
States.
Figure 4 illustrates one such system used in a small
restaurant,
uecf4x13.gif (600x600)

in New Delhi, India.
During the hottest part of the day the
owner of the restaurant would first start the water pump,
and
wait for the coconut mat to become soaked with water.
After
this, the fan would be turned on to force hot dry air
through the
water-soaked mat.
The thickness and density of the mat were
sufficient to slow the speed of the air and permit enough
evaporation
to cool the air substantially.
This air was, in fact,
cool enough to keep people from sitting close to the cooler
for
even short periods of time.
Even though this cooler is very effective at cooling room
air, it
has several important disadvantages.
First, this system depends
on electricity to power both the water pump and the
fan. Second,
the cool air that is blown into the room has a relative
humidity
of nearly 100 percent.
In some situations this high level of
humidity may be an undesirable since it may promote the
growth of
mold and mildew. The
small restaurant in India that used this
system avoided this problem by having only part of the
restaurant
covered by a roof.
This allowed the saturated air to quickly
escape outdoors. A
further disadvantage of this method is its
constant consumption of water.
In areas where water is in short
supply, its use for cooling purposes may not be justified.
Despite these disadvantages, this cooler is capable of
cooling an
indoor area at a fraction of the cost of a commercial
refrigerated
air conditioning system.
Cabinet Produce Coolers
Large amounts of fresh produce and dairy products are lost
due to
spoilage in many tropical and subtropical areas of the
world. If
this food could be stored at relatively low temperatures
until
eaten or sold, much of this waste could be avoided.
For many of
these areas, though, commercial methods of cooling food are
either unavailable or too expensive.
Evaporative cooling may be a
practical alternative for use in tropical and subtropical
regions.
There are several types of cabinet coolers that use the
principles
of evaporative cooling to cool stored produce.
Four types of
cabinet coolers are described below, in order of increasing
complexity.
Type I Cooler
This simple cooler (Figures 5 and 6), which is essentially a
uecf5150.gif (540x540)

variety of materials ranging from bamboo to sawed
timber. It can
be cylinderal or rectangular in shape.
The cloth covering
(Figure 6) that surrounds the cabinet cooler absorbs water
from
uecf6x16.gif (600x600)

the troughs at the top of the base.
Eventually the entire cloth
becomes soaked with water, and as the air moves past the wet
cloth, evaporation occurs.
As long as evaporation takes place,
the contents of the cabinet will be kept at a temperature
lower
than that of the environment.
Under certain conditions, this simple cooler may be unable
to
maintain low temperatures.
For example, if the air is very dry
and the wind very brisk, the drying action may exceed the
absorbing
action of the cloth, thus preventing it from staying moist.
This in turn will prevent the cooler from achieving and
maintaining
a temperature much lower than the environment's.
This
type of cooler requires periodic attention to refill the
water
troughs, which may be a problem.
The consumption of water may
also pose a problem for areas where water is either scarce
or
difficult to obtain.
The major advantages of this cooler are its relative
simplicity,
low construction costs, and independence from commercial
energy.
Type II Cooler
The Type II cooler was designed to eliminate some of the
problems
associated with the Type I cooler.
The design of the Type II
cooler is much the same as the Type I cooler, except that
the
walls of the Type II cooler are thicker and the water trough
is
replaced by containers of water that are positioned on top
of the
cooler.
The walls can be constructed from a variety of materials as
long
they meet the following requirements:
(1) the material must
allow air circulation; (2) it must very absorbent and
capable of
holding a substantial amount of moisture; and (3) the
material
itself, or the frame surrounding it, must be strong enough to
support the containers of water that will sit on top of the
cooler. One of the
walls of the cooler also functions as a door.
Inside the cooler, lattice shelves are spaced wide enough
apart
so that there is as little obstruction to the air flow as
possible.
Small holes are punched along the outer edge of the bottom
of the
water containers.
This allows the water to drip slowly down to
the absorbent wall material.
The drip flow should be fast enough
to keep the walls continually moist, but not so fast as to
allow
water to drip out of the bottom of the cooler.
Obtaining the exact
rate of flow requires some experimentation, but with
patience, an optimal flow rate can be achieved.
One such cooler (Figure 7) was built by the author for use
in
uecf7x18.gif (600x600)
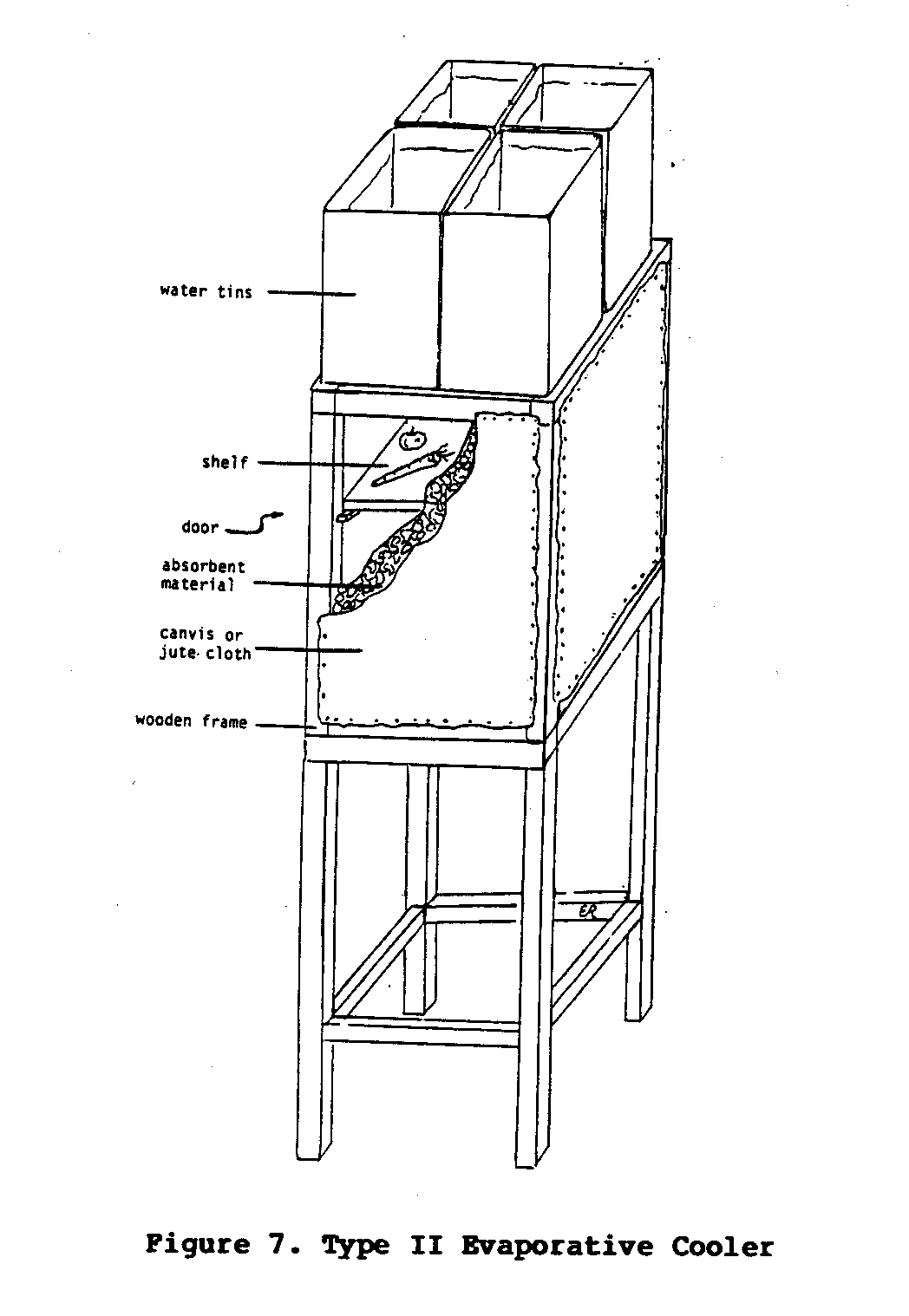
eastern Kenya. Four
"debi tins" (these are rectangular containers,
were originally used to store and transport biscuits)
each with an eight-liter capacity, were used as water
containers.
The holes were first punched in the bottom of the
containers,
about 0.5 centimters apart, using a nail.
Each hole was then
filled with candle wax which was punctured with a small
needle.
The wax allowed for the experimentation necessary to achieve
the
proper size holes for the optimum rate of water flow.
The absorbent walls of this cooler were made by first
attaching
sheets of jute cloth on either side of a rectangular wooden
frame
made from five centimeters by five centimeters lengths of
timber.
Next, small mesh chicken wire was tacked over the jute
cloth.
From a notch cut through the top of the frame, small chunks
(approximately 0.5 centimeters in diameter) of charcoal were
poured into the frame and packed between the sheets of jute
cloth. The chicken
wire helped to keep the walls from bulging.
The combination of jute cloth and charcoal allowed
sufficient air
flow to permit evaporation, while at the same time allowing
the
wall material to remain soaked with water.
On very hot, dry, and windy days, the four containers of
water
usually lasted the entire day.
At the end of cooler, less windy
days, the containers would often be found partially filled
with
water. The remaining
water then be poured into a container and
saved for the next day.
Fruits and vegetables were the primary foods kept in the
cooler,
but occasionally milk and meat were also stored for short
periods
of time. The
reduction in temperature achieved by this cooler,
along with the high level of humidity, were sufficient to
allow
the storage of most fruits and vegetables for five to ten
days,
and sometimes even longer.
Vegetables that were stored in a
shaded area would usually spoil in only two or three
days. Milk
or meat that was placed in the cooler in the morning would
usually
be fresh in the evening when it was needed for the evening
meal. When not
stored in the cooler, milk and meat would usually
be spoiled by mid-afternoon.
Drinking water was also kept in the
cooler. This
provided a much more satisfying and refreshing
drink than water kept in bottles placed either under trees
or in
the house.
On days when there was little or no wind, or when the
humidity
was high, the temperature in the cooler was not much less
than
the environment's.
However, for most situations in eastern
Kenya, this cooler prevented a substantial amount of food
from
spoiling and provided cool water for drinking.
The Type II cooler requires a some carpentry skill to build
and
tools such as a saw, hammer, block plane, and chisels.
Additionally,
the author used sawn timber, but it may be possible
to use other materials and achieve a similar degree of
efficiency.
Even though charcoal and jute proved to be very effective
materials for the cooler's walls, similar material could be
substituted.
Consideration needs to be given to the probable
effectiveness of evaporative cooling for the specific
environment
under question before this cooler is built.
Type III Cooler
This third type of evaporative cooler, often referred to as
the
Janatha air cooler, was originally designed and built in
India
using baked clay building blocks called "Hourdis"
block (Figure 8).
uecf8x20.gif (486x486)

These blocks, are stacked together to form a rectangular
three-walled enclosure.
Slotted or grilled shelves are arranged
in the cooler and a wooden top and door seal the
structure. The
cooler is usually built on a cement platform.
The hollow core of
each of the clay building blocks is kept filled with
water. This
water slowly seeps through the porous clay walls of the
Hourdis
block, eventually evaporating from the surface, thus cooling
the
entire structure.
Small holes are often drilled in the sides of
each of the blocks and fitted with short lengths of pipe
that
connect all of the hollow water-filled blocks together.
From one
of the blocks another short length of pipe is fitted to
extend
outside the cooler.
This pipe is used to drain the cooler
periodically to prevent a buildup of salt and mineral
deposits in
the pores of the baked clay.
If the cooler is not drained, the
flow of water through the pores of the clay will eventually
stop.
A diagram of a completed Type III cooler is illustrated in
Figure 9.
uecf9x21.gif (600x600)

Two graduate engineering students at the University of Texas
designed an evaporative cooler similar to the Janatha air
cooler.
Instead of using baked clay, which is known to have a
relatively
low level of porosity (i.e., the ability of water to flow
through
the small pores present in a material), the students used
blocks
made from jute cloth saturated with a very watery cement
mixture.
Before the cement dries and sets, the dip-molded blocks can
be
formed into desired shapes.
This process of dip-molding allowed
the experimenters to build large blocks that not only had a
high
level of porosity, but were also very strong and relatively
light. Using this
technology, the students built a cooler that
used long tubular blocks (Figure 10).
uec10x23.gif (600x600)

Other experiments with dip-molded blocks indicated that a
single
block could be shaped directly into the walls of the cooler
(Figure 11). An
experimental U-shaped cooler is shown in Figure 12.
uec11x24.gif (600x600)

uec12x25.gif (600x600)

Type IV Cooler
This final type of cooler uses electricity to power both a
small
fan and in some cases a small water pump.
Essentially, this is a
small version of the indoor curtain cooler described
earlier. It
can either be designed and built to be a permanent structure
or
it can be made as a portable unit.
If a permanent cooler is
desired, it can be built along the lines of the Type II
cooler.
Since a fan is used, the rate of air flow can be regulated
to
achieve an optimum rate.
Moreover, the rate of evaporation and
therefore cooling will be rapid since these systems are not
at
the mercy of intermittent winds.
There are variations of this
cooling system: (1)
an electrified version of Type II cooler,
and (2) a portable electric cooler.
The efficiency of the Type II cooler can be improved with
the
addition of a small fan and water pump.
The fan can either be
placed in the door or near the bottom of the cooler.
The action
of the fan draws air through the water-soaked walls of the
cooler
at a constant and even rate.
This air, cooled through evaporation,
cools the food and water stored in the cooler.
The containers of water used in the Type II cooler are
replaced
with small troughs positioned along the upper and lower
edges of
the cooler. The
constant circulation of water ensures that the
----------------------
(*) A detailed description of dip-molding can be found in
the report
by W. Hutchinson and R. Chuang, Inexpensive Evaporative
Coolers
for Short-Term Storage of Fruits and Vegetables: A Design
Study
Report (See Bibliography).
absorbent wall material is always soaked with water.
The troughs
along the bottom of the cooler should be built large enough
to
hold enough water for a full day's cooling.
The second form of the Type IV cooler is an electric
portable
cooler. One such
portable cooler was designed and built by two
researchers at the University of California.
Even though this
portable evaporative cooler was intended to be used
primarily by
fruit growers in the Southwestern United States, it should
also
prove useful to individuals living throughout tropical and
subtropical
areas of the world.
Basically, this portable cooler is a simplified,
single-walled
version of the electrified Type II cooler.
As shown in Figure 13,
uec13x27.gif (600x600)
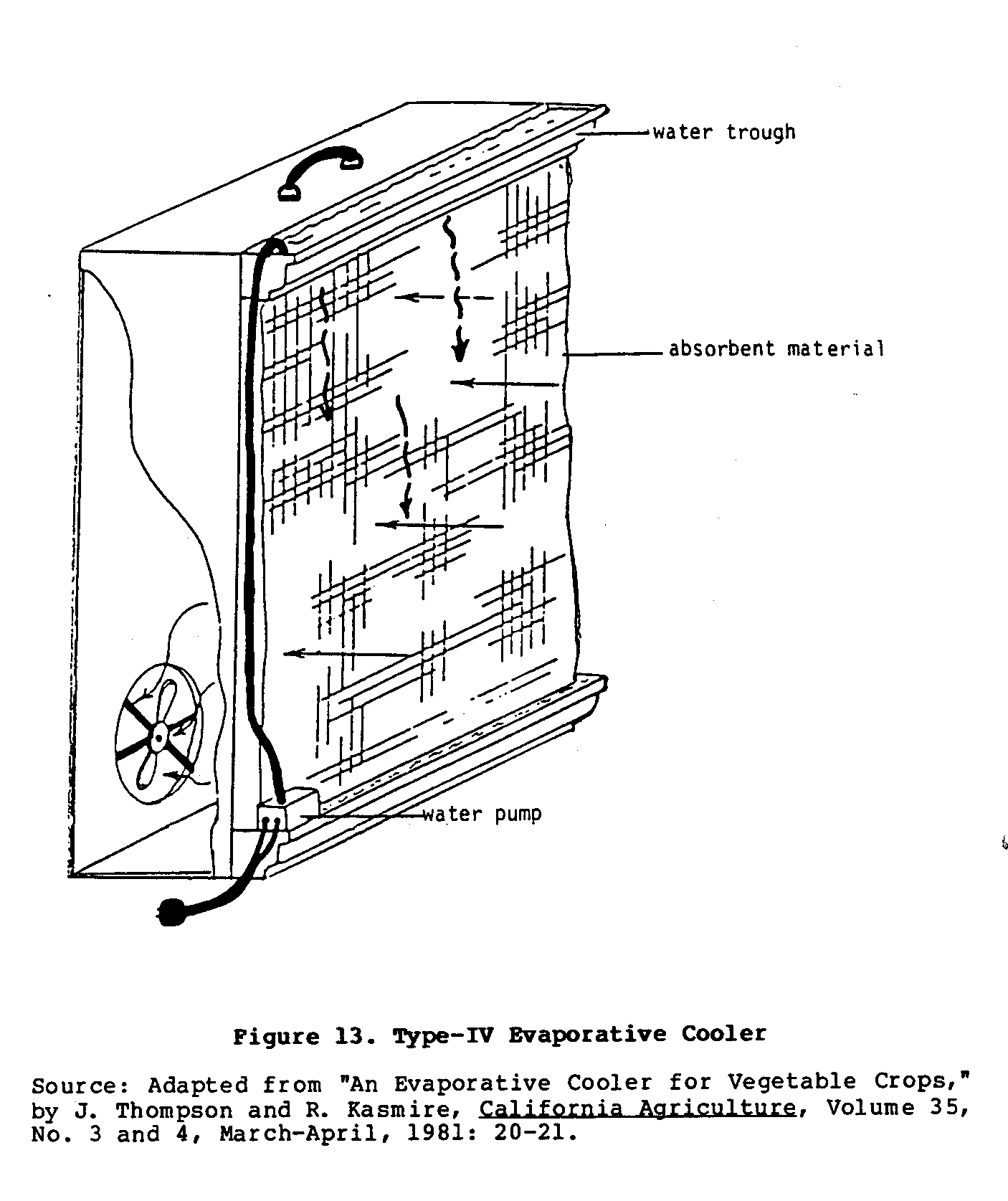
one wall is a sheet of absorbent material, while the
opposite
wall has a fan attached to it.
Small troughs above and below the
wall of absorbent material hold water.
A drainage hose from the
lower trough is connected through a small water pump to the
trough on top of the cooler.
This provides constant circulation
of water through the system.
Boxes of fruits and vegetables are placed around the
portable
cooling unit. The
fan forces cool moist air past the produce in
the boxes. Figure 14
illustrates an example of this set-up.
uec14x28.gif (600x600)

Slowly, the freshly picked produce will be cooled to
temperatures
that will promote optimum storage life.
This portable cooler has been designed to prevent produce
from
spoiling before it is sold or sent to market.
Since this unit
takes up very little space and consumes so little
electricity,
many fruit and vegetable vendors throughout the tropics may
find
this cooler a cost-effective method of protecting their
valuable
merchandise.
INDIRECT EVAPORATIVE COOLING
The high level of humidity that is produced by direct
evaporative
cooling may be undesirable for some applications.
Indirect evaporative
cooling attempts to solve this problem by using the cool
moist air produced through evaporation to cool drier
air. The
resulting cool dry air is then used to cool the desired
environment.
This transfer of coolness is accomplished with the help of
a heat exchanger.
All methods of indirect evaporative cooling require power to
run
both water pumps and fans.
For this reason, indirect evaporative
cooling will have limited application.
It is primarily used to
cool dwellings and rooms.
In such situations these cooling systems
are generally less expensive to buy or build and operate
than conventional air conditioning systems.
On the other hand,
indirect evaporative cooling cannot be used in all environments,
and the reduction in temperature that can be achieved with
this
system is not as great as the reduction that can be achieved
with
conventional mechanical cooling systems.
Basic Characteristics of a Beat Exchanger
Figure 15 is a simplified diagram of a heat exchanger.
The heat
uec15x30.gif (600x600)
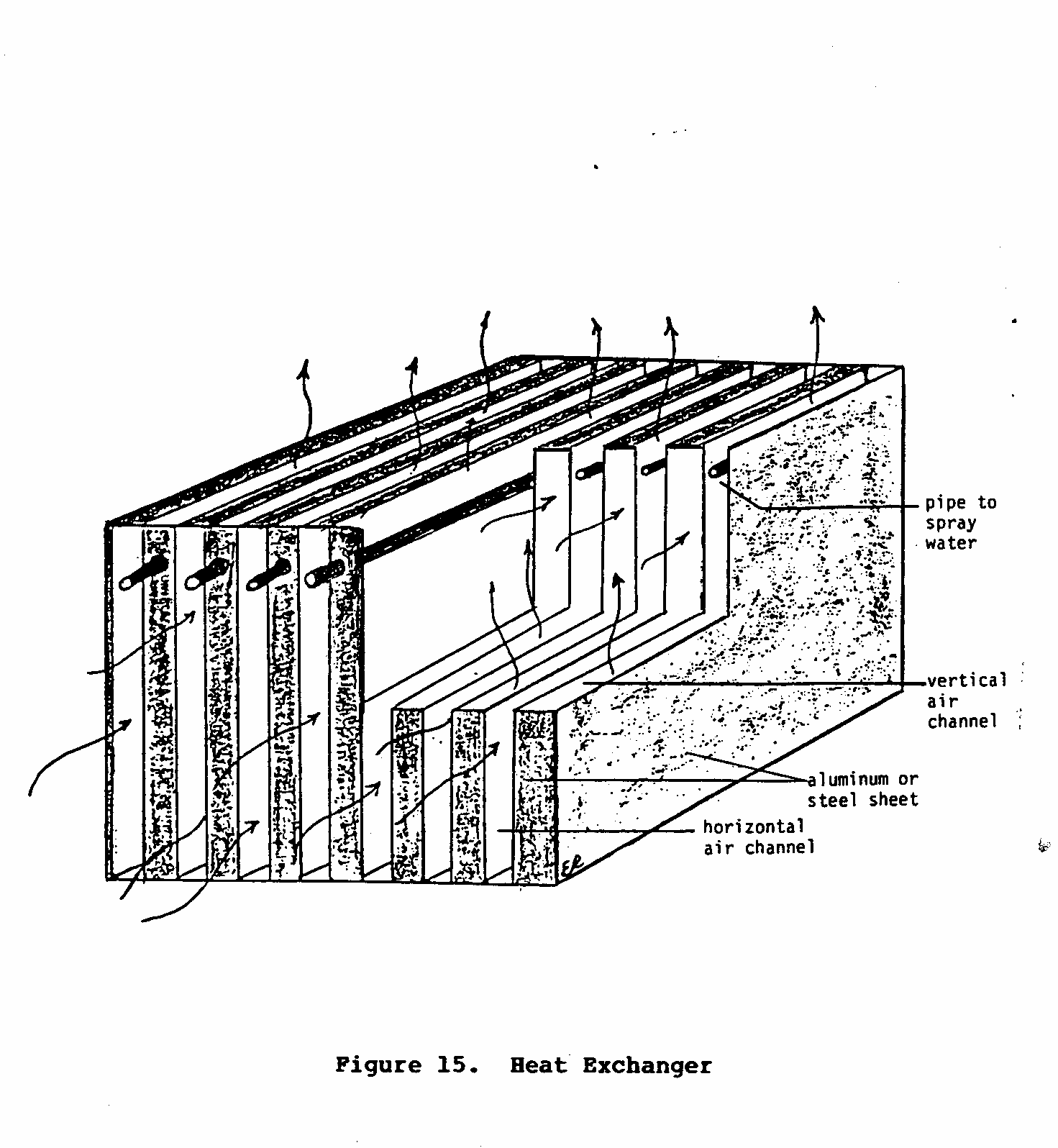
exchanger is composed of two sets of alternating channels
through
which air flows. The
air that passes through the vertical
channels comes in contact with water that is either being
sprayed
or dripped into the channel.
If this air is warm and dry,
evaporation and cooling will occur.
This cool air then cools the
channel walls, which in turn cools the air that is being
forced through the horizontal set of channels.
Finally, the cool
moist air is directed outside the dwelling, while the cool
dry
air is blown into the room or building that needs to be
cooled.
Factors That Effect Cooler's Effectiveness
As with direct evaporative cooling, several factors
influence the
effectiveness of this cooling system.
Among the most important
are the relative humidity and the temperature of the air
being
cooled. Low levels
of relative humidity promote rapid evaporation
and, therefore, a greater rate of cooling can be achieved.
The rate of evaporation will also be increased if the air
temperature
is relatively high.
Incoming air with a high temperature,
however, will need more cooling than cooler air; therefore,
high temperatures can be both an advantage and a
disadvantage.
Two other factors that also affect the rate of cooling are
the
rate of air flow through the heat exchanger and the
character of
the water that is used in the evaporative cooling
process. If
the air is forced through the heat exchanger too quickly,
little
evaporation will take place, and therefore, little cooling
will
occur. Air
turbulence within the channels may increase the rate
of evaporation. The
size of the water droplets will also
influence the rate of cooling since it will have a
significant
affect on the rate of evaporation.
If the water droplets are
large, they will have a relatively small total surface area,
compared to their volume, from which molecules of water can
evaporate. Smaller
droplets have a greater surface area,
compared to their volume, and therefore, evaporation will
occur
more rapidly. This
will in turn promote rapid cooling.
Finally,
the temperature of the water being sprayed or dripped into
the
channels will also affect the efficiency of the cooler.
If the
water is cold, the walls of the heat exchanger will cool
down
quickly. However,
this may also slow down the rate of evaporation
since cool droplets need to absorb more energy before
evaporation
occurs.
The design of the heat exchanger will also influence the
rate at
which cooling occurs.
For example, small channel spaces will
promote more rapid cooling than larger, more spacious
channels.
Moreover, if the heat exchanger is made from a material that
conducts heat efficiently, such as metal, the transfer of
coolness
from the wet channels to the dry ones will occur more
effectively.
Two Examples of Indirect Cooling Systems
There are two types of indirect evaporative cooling
systems. The
basic difference between these two systems is in the design
of
their heat exchangers.
In one system, air is circulated through
the heat exchanger in both horizontal and vertical
directions
(bidirectional). The
air forced through the vertical set of
channels will be [used to cool] the air flowing through the
horizontal set of channels.
The air in the horizontal channels
remains dry and will be used to cool the room.
In the second
system, air flows through both sets of channels in the same
direction, but like the first system, the cool dry air is
released
into the room while the cool moist air is directed outside.
Forcing air through the heat exchanger in two different
directions (Figure 15) has the advantage of being able to
use two
different sources of air.
For example, the air for evaporative
cooling can be taken in from the room, while the air that is
used
to cool the room can be taken from the outside.
Figures 16 and 17 sketch the basic characteristics of one
such
uec16320.gif (600x600)

and the design of the heat exchanger and cooler can vary
significantly
depending upon the materials used and the skill of the
builder. Figure 16
shows the two different air circulation
uec16x32.gif (600x600)
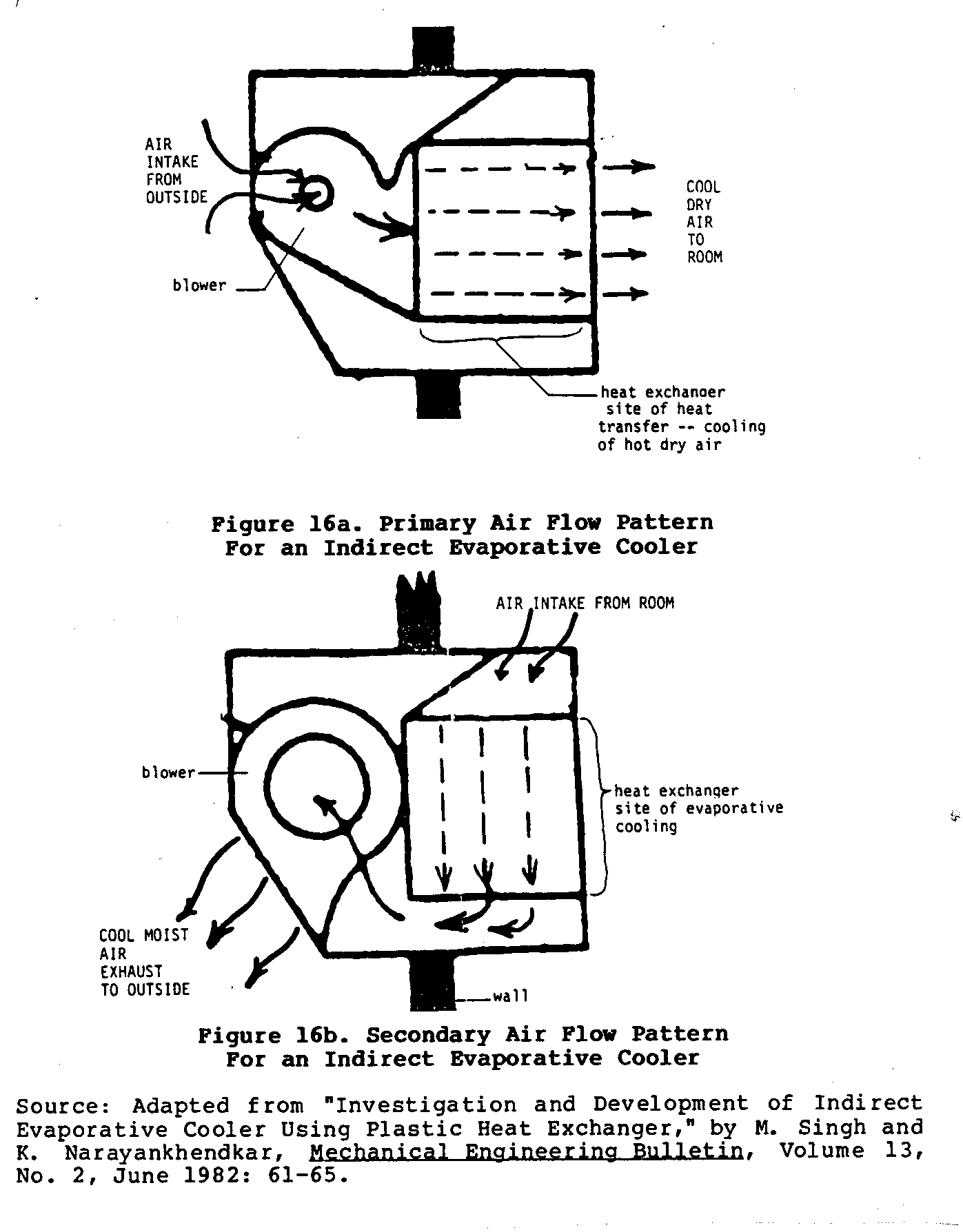
patterns mentioned earlier.
Figure 17 shows four different views
uec17x33.gif (600x600)

of a working model of bidirectional cooler.
This type of cooler
uses two blowers to achieve this bidirectional flow of air.
Most heat exchangers are made of metal, but a mass-produced
plastic heat exchanger was used successfully in an indirect
evaporative cooler in India.
No matter what type of heat
exchanger is used, it be important that it be designed and
built
to take advantage of the various principles that can
positively
influence evaporation and heat transfer.
The primary advantages of indirect evaporative cooling for
increasing the comfort level of rooms are the relatively low
purchase or building cost and the relatively low operation
expense, as compared with conventional air conditioning
systems.
Before deciding upon indirect evaporative cooling, though,
it is
important that the necessary environmental conditions,
discussed
earlier, be present. The more favorable these conditions
are, the
more effective the cooler will operate. One such cooler,
developed
in Baghdad, Iraq, proved to be a practical alternative to
conventional mechanical air conditioners. This cooler
produced
seven times the cooling was a conventional air conditioner,
while
consuming the same amount of electricity. This greater
effectiveness
was in part due to the 17[degrees] centigrade average
difference
between the wet- and dry-bulb temperatures common in
Baghdad.
IV. COMPARING THE ALTERNATIVES
The principal alternatives to evaporative cooling systems
are
refrigeration and air conditioning. These technologies offer
the
user a much wider range of application. If electricity,
(including that produced by photovoltaic cells), natural
gas, or
kerosene are available, commercial refrigeration and air
conditioning systems can be used in any environment
regardless of
th temperature or relative humidity. This is definitely not
the
case with evaporative cooling. Moreover, commercial systems
allow
the user to control the amount of cooling desired. Again,
this
is not possible with most evaporative cooling systems.
Another
advantage of commercial systems is that they usually require
less
day to day attention than comparative evaporative cooling
systems. However, where electricity or other commercial
energy
sources are either unavailable or very expensive, and the
environmental
conditions are favorable, evaporative cooling should be
considered as a viable alternative to these more complex and
costly commercial systems.
Although lowering the temperature of fruits and vegetables
to
retard spoilage is an important benefit of evaporative
cooling,
it is not the only one. Evaporation not only lowers the air
temperature surrounding the produce, it also increases the
moisture content of the air. This helps prevent the drying
out
of produce, and therefore extends its shelf life.
The primary advantage of evaporative cooling over cooling
methods
that involve commercial refrigeration is its low cost. For
example, an evaporative cooling system developed in the
United
States to cool fresh produce was able to produce 14 energy
units
of cooling while using only one energy unit of electricity.
Commercial refrigeration systems commonly produce only three
energy units of cooling for each energy unit of electricity
consumed. Low operating costs in addition to low purchase or
construction costs substantially reduce the total cost of
cooling
by evaporation.
One final alternative deserves mention. It is possible to
produce
ice at night, even if the air temperature is above the
freezing point, if certain specific conditions are met. This
cooling and freezing is accomplished though the joint
processes
of radiation and evaporation and could be used to produce
ice for
cooling. To be effective, natural freezing requires
appropriate levels
of humidity, clear unobscured skies, and little or no
wind. Arid environments usually offer such conditions.
To produce ice this way all that is needed is a large flat
container that has a clear view of the sky and is well
insulated
from the ground. Figure 19 shows one such set-up that
regularly
uec19x36.gif (600x600)

produced ice for a reseacher at Purdue University in the
United
States. This device was placed in a field away from all
trees
and buildings and filled with 2 - 3 centimeters of water. On
nights with temperatures between 4 and 7[degrees]C and with
relative
humidities of 90 - 100 percent about 7.5M of ice would form
on
the surface of the water. If not collected and stored in an
insulated cooler early in the morning, the ice would quickly
melt
soon after the sun rose. It is possible that enough ice to
cool
food for a 24- to 48- hour period could be produced using
this
process if a large enough natural freezer was used.
The chief disadvantage of this system is its dependence upon
a
narrow set of environmental conditions, and a corresponding
lack of reliability. The graphs in Figures 20 and 21 show
how
uec20x37.gif (600x600)
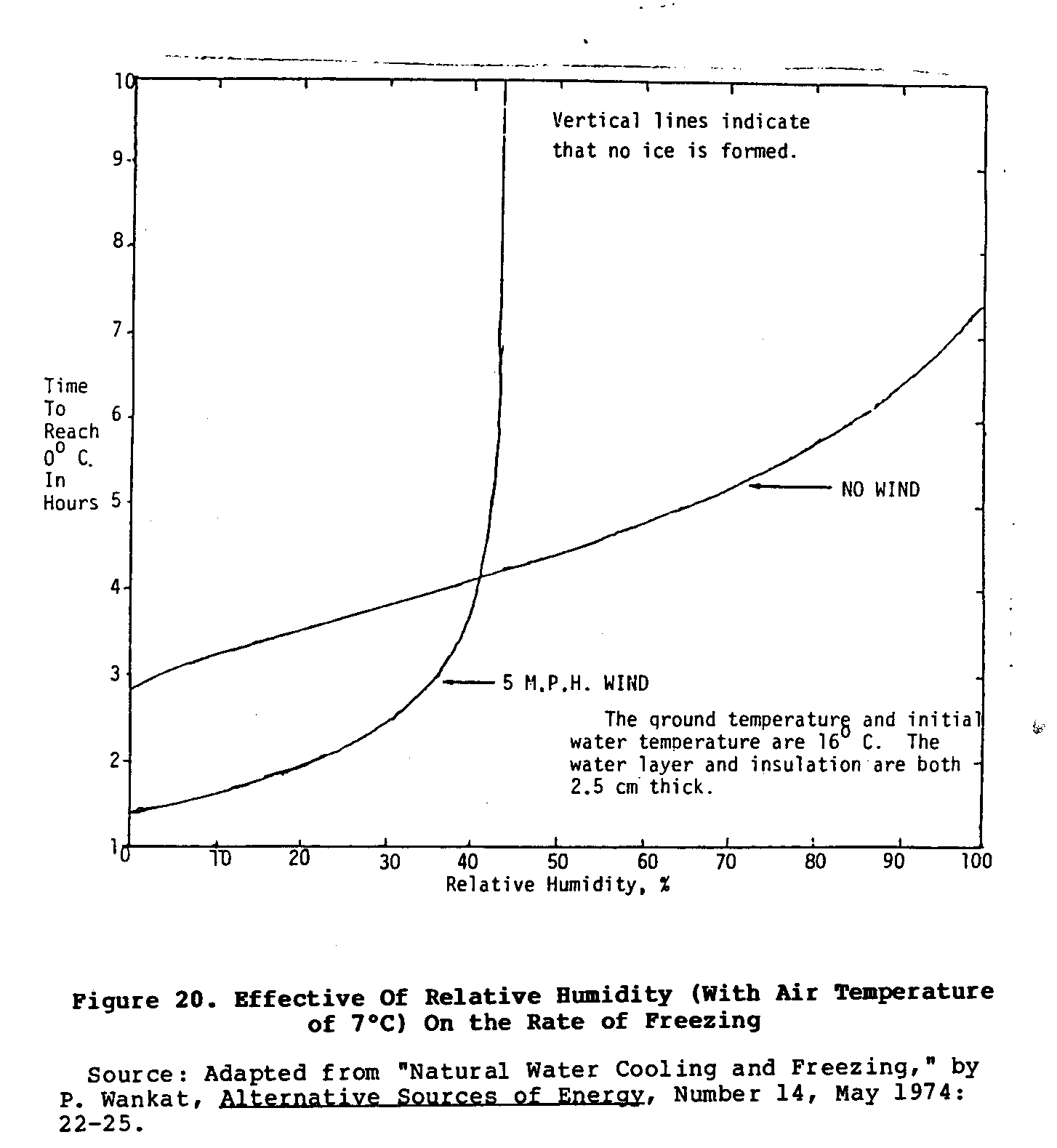
wind, air temperature, and relative humidity affect the rate
of
cooling of this natural freezer. Moreover, if the night is
not
perfectly clear, the rate of cooling is reduced. This system
also requires the user to wake up before the sun rises to
collect
and store the ice that may have formed during the night. If
little or no ice formed because of poor conditions, the user
would be unable to cool stored food. However, if ice is
needed
only occasionally, this is an inexpensive method of making
it.
V. CHOOSING THE TECHNOLOGY RIGHT FOR YOU
Making a decision on which type of cooling or refrigeration
system to use is not an easy process. It is important to
review
carefully the cooling needs, weighing them against a range
of
other factors, before selecting any of the options discussed
in
this paper. If this is not done, frustration and
disappointment
may result. <see figure 18>
uec18x36.gif (486x486)

The following checklist may be useful in choosing a suitable
technology. Since every situation is different, this
checklist
may not always apply, but it should be of some help.
1. What are your cooling needs? Cooling different foods
requires
different
temperatures. Cooling rooms or buildings is different
from cooling food.
2. What is the average relative humidity of the area where
cool-is
needed? If the
relative humidity is consistently high,
evaporative
cooling will not be available option, and therefore
another system
needs to be considered. If the relative
humidity is low,
then evaporative cooling may be very effective.
3. How windy is the area where the cooling is needed? If
there is little
wind, evaporative cooling may not be the way
to go.
4. Is there a good supply of water where the cooling system
will be used? If
water is readily available, evaporative cooling
may be feasible.
5. Are the materials and skills needed to build the cooler
available?
6. Is electricity available? Is it very costly? If
electricity is
available and
affordable, then a powered evaporative cooler
may be the best
choice since it offers more freedom and is
generally more
effective than passive evaporative cooling systems.
7. Are commercial mechanical cooling or refrigeration
systems
available? Are
they costly? If commercial systems are available,
and not too
costly, then they may be a better choice of
technology.
The design and construction of some of the evaporative
coolers
discussed in this paper may require the investment of a
substantial amount of time and money. It may, therefore, be
advantageous to turn the building of the evaporative cooler
into
a business. In India, for example, a local town builder has
started a business building Janatha air coolers. Before this
is
done, however, it should be determined if there will be
sufficient demand for such a cooler to warrent setting up a
business.
If only a few individuals want to buy or build evaporative
coolers
it may be possible to build the coolers. By buying necessary
parts in volume, and by contracting out for the actual
construction,
the group can reduce the cost per cooler. As with all
cooperative efforts, it is important to keep very accurate
records
of all transactions.
REFERENCES
1. "A Village
Food Cooler", AP-Tech Newsletter, July 1980,
Volume 4, No. 1,
pp. 10-11.
2. Akuffo, F.O. and
K.D. Klorbortu, "Experiments on Food
Storage in the
Tropics Using Evaporative Cooling", VITA
Document No.
VIII-F-2; 013594.
3. Dunkle, R.V.,
"A Method of Solar Air Conditioning:, Mechancal
and Chemical
Engineering-Transactions-of The Institution
of Engineers,
Australia, Volume 7, No. 3, September,
1984, pp. 1-2.
4. Exell, R.H.B.
"Solar Absorption Refrigerators in AIT",
RERIC News,
Bangkok, Thailand, Volume 7, No. 3, September
1984, pp. 1-2.
5. Hutchinson, Bill
and Roger Chuang Inexpensive Evaporative
Coolers For Short
Term Storage of Fruits and Vegetables: A
Design Study
Report. Mechanical Engineering Department, The
University of
Texas at Arlington. May 1976, VITA Document
No.
VIII-F-2-003317.
6. Latif, Abbas A.
and Nabeel A. Mahmood. "Indirect Evaporative
Cooling",
ASHRAE Journal, January 1968, pp. 61-67.
7. Relative Humidity
Tables, (prepared by) The Consumer Products
of Sybron
Corporation, Adren, North Carolina.
8. Singh, Mastinder
and K.G. Narayankhendkar. "Investigation and
Development of
Indirect Evaporative Cooling Using Plastic
Heat
Exchanger", Mechanical Engineering Bulletin, Volume 13,
No. 2, June 1982,
pp. 61-65.
9. Thompson, James
F. and Robert F. Kasmire. "An Evaporative
Cooler for
Vegetable Crops", California Agriculture, Volume
35, No. 3 and 4, March-April 1981, pp.
20-21.
10. Thompson, James F. and Robert F. Kasmire. "An
Evaporative
Cooler for
Vegetable Crops", California Agriculture, Volumne
35, No. 3 and 4,
March-April 1981, pp. 20-21.
11. Wankat, Philip. "Natural Water Cooling and
Freezing:, Alternative
Sources of
Energy, No. 14, May 1974, pp. 22-25.
========================================
========================================

