Biogas originates from bacteria in the process of biodegradation of organic material under anaerobic conditions. It consists of a varying proportion of CH4 (methane) and CO2 (carbon dioxide) and traces of H2S, N, CO, O, etc. The content of CH4 and CO2 is a function of the matter digested and the process conditions like temperature, C/N ratio, etc. Methane is the most valuable component under the aspect of using biogas as a fuel; the other components do not contribute to the calorific ("heating") value and are often "washed out" in purification plants in order to obtain a gas with almost 100% CH4. For further details of biogas production the use of the respective literature is recommended [3, 4, 5, 6].
The useful part of the energy of biogas is the calorific value of its CH4 content. The other components have strictly speaking an energy content also but they do not participate in a combustion process. Instead of contributing they rather absorb energy from the combustion of CH4 as they usually leave a process at a higher temperature (exhaust) than the one they had before the process (mainly ambient temperature).
The following are the thermodynamic parameters of CH4 at standard conditions¹ (i.e. 273 K, 1013 mbar=0.1013 MPa):
- specific treat cp = 2.165 kJ/kg K,
- molar mass
M = 16.04 kg/krnol,
- density r =0.72
kg/m³,
- individual gas constant R =0.518 kJ/kg·K,
- lower
calorific value
Hu = 50000 kJ/kg,
Hu,n = 36000
kJ/m³n.
The actual calorific value of the biogas is a function of the CH4 percentage, the temperature and the absolute pressure, all of which differ from case to case. The calorific value of the biogas is a vital parameter for the performance of an engine, a burner or any other application using biogas as a fuel. The calculation of the calorific value can be done using the standard thermodynamic relations for gases:
-Standard gas equation
p·V = m·r·T (Equ. 4.1)
-isentropic exponent
g =
cp/cv (Equ. 4.2)
-specific gas constant
R = cp-cv (Equ.
4.3)
-constant volume process(v=constant)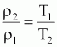
- constant pressure process (p = constant)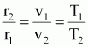
- constant temperature process (T = constant)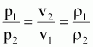
Fig. 4.1: Density r of CH4 as a function of biogas pressure
and temperature
Fig. 4.2: Calorific value of biogas
as a function of the density and volume %-age of its CH4 content
(r=0.72 is the density at a standard condition)
The graphs (Figs. 4.1, 4.2) will facilitate an easy determination of the density of the CH4 component in a first step and the calorific value of the biogas in a second step. Use the diagrams as follows:
-Determine the actual density r of the CH4 in the biogas using the actual biogas temperature and pressure (ambient pressure + biogas plant pressure (gauge) or pressure measured at inlet to the mixing device).
- Find the actual calorific value using the density and the percentage of CH4 in the biogas mixture.
A precise calculation of the calorific value can be done following the example below.
Example:
Calculation of the calorific value of biogas at the
following conditions:
-composition:
CH4 = 60% Vol, i.e. VCH4/Vtot =
0.6
CO2 = 40% Vol, i.e. VCO2/Vtot = 0.4
Traces of other components negligible
|
-temperature: |
T = 298 K (= 25 °C) |
|
-pressure, ambient: |
Pa = 950 mbar |
|
-pressure in biogas plant: |
pp = 20 mbar, gauge |
Step 1: total pressure of biogas
Pt = 950 + 20 =
970 mbar 
If humidity of biogas was not considered in the gas analysis so far, the value has to be corrected using the diagram in Fig. 4.3 and the related example.
Step 2:. density r of CH4 in mixture at actual pressure p and temperature T, calculated on the basis of the table values at standard conditions
|
- temperature correction: |
|
|
- pressure correction: |
|
- 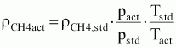
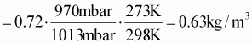
Step 3: actual calorific value of given biogas
- 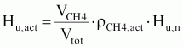
= 0.6·. 0.63 kg/m³· 50 000 kJ/kg
=
18900kJ/m³
Compare with value obtained when using the diagrams in Figs. 4.1 and 4.2.
Biogas emerging from the plant is usually fully saturated with water vapor, i.e. has a relative humidity of 100%. Depending on the course of the gas piping between plant and consumer, part of the water vapor will condense when the gas is cooled. The humidity can be reduced by cooling and warming again of the gas with a drain trap for the condensate at the cooler.
The gas analysis often either does not consider the humidity or it is done at the plant, not at the consumer. In those cases the humidity needs to be considered for the establishment of the calorific value. This can be done by subtraction of the partial pressure p' of the water vapor from the total gas pressure pt. The remainder is the corrected pressure value pc to be considered in the above calculations of the calorific value.
pc = pt-p' (Equ. 4.9)
The partial pressure of water vapour itself is a function of the gas temperature and the relative humidity as given in Fig. 4.3.
Example:
given:
|
- gas temperature: |
tg = 40ºC |
|
- relative humidity: |
100% |
|
- total gas pressure: |
pt = 970 mbar |
Fig. 4.3: Partial pressure of water
vapor in a mixture with biogas as a function of a biogas temperature and
relative humidity
Solution:
Step 1: partial pressure from diagram: p' = 70 mbar
Step 2: corrected gas pressure for calculation of calorific value from Step 1 in previous example onwards:
pc = pt - p' = 970 - 70 = 900 mbar
The fuel consumption of equipment using biogas is often specified in m³n/h or m³n/kWh, i.e. standard cubic meters per hour or per kilowatt hour (sic) respectively. The standard cubic meter (m³ n) means a volume of 1 cubic meter of gas under standard conditions, i.e. at a temperature of 0 °C (273 K) and a pressure of 1013 mbar. The consumption of biogas in actual volume will differ from these data according to the actual conditions of the biogas as fed to the equipment (motor, burner, etc.) in terms of
-temperature,
-pressure,
-composition, i.e. CH4
content.
The determination of the actual volumetric consumption of an engine operating on biogas fuel is of utmost importance for the dimensioning of biogas plant, engine, mixing device and other equipment. A difference of 50% between actual volumetric consumption and specified consumption of a biogas engine can easily occur and could result in poor performance of the engine if not considered.
Using the diagrams Figs 4.1 and 4.2 the consumption of the specific biogas can easily be found:
Step 1:
Check how the fuel consumption fc is specified.
-If in m³n/h, continue with step 2.
-If in m³/h
without biogas specification assume a calorific value of Hu = 20 000
kJ/ m³ .
-If as specific fuel consumption at rated conditions use fc =
sfc· P (in m³/h). (Equ. 4.10)
-If only the efficiency h is specified use fc = 1/h·p·1/Hu· 3600 (in
m³/h). (Equ. 4.11)
-If no information is given use Equ. 4.11 with h= 0.3 for dual fuel and larger Otto gas engines and
h = 0.25 for smaller Otto gas engines as well as
Hu = 20 000 kJ/m³.
Step 2:
Determine the calorific value of the biogas used for specification of the equipment by the manufacturer.
- If consumption is specified by engine supplier in kJ/h, use
this value and continue further below in step 4.
- If calorific value of
biogas is specified in kWh/m³ n transform this figure by multiplying by
3600 to obtain it in kJ/m³ n.
- If biogas is specified by its CH4
content in Vol % use diagrams in Figs. 4.1 and 4.2 to obtain the calorific value
in kJ/m³ n.
Step 3:
Determine the required energy flow (calorific consumption) of the engine at rated performance in kJ/h by multiplying the specified consumption rate at standard conditions in m³ n/h with the calorific value of the biogas in kJ/m³ n, as-specified by the engine supplier (energy consumption = specified volumetric consumption x calorific value of biogas).
Step 4:
Determine the actual calorific value of your specific biogas in kJ/m³ using the procedure explained in Chapter 4.2.
Step 5:
Determine how much of your specific biogas will be consumed by the engine in m³/h by dividing the energy consumption (Step 3) by the calorific value of your specific biogas (Step 4):

Example:
Manufacturer's engine specification:
- power rating P = 20 kW
- fuel consumption at rated power fc
= 10 m³ n/h
- biogas used 70% CH4, 30% CO2
Specification of biogas from your plant (see Chapter 4.2)
Hu = 18 900 kJ/m³
Step 1:
No calculation needed as the fuel consumption is specified.
Step 2:
From diagram Fig. 4.2 calorific value of biogas used in specification of manufacturer:
Hu,n = 25200 kJ/m³ n (at standard conditions).
Step 3:
Energy consumption (flow) of the engine at rated power
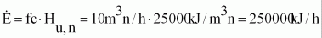
Step 4:
Calorific value of your specific biogas from plant (see Chapter 4.2)
Hu = 18 900 kJ/m³.
Step 5:
Actual biogas consumption fc of engine at rated power
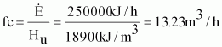
The volumetric fuel consumption in this case would be 32% higher than specified by the manufacturer at standard ("n") conditions, which demonstrates that the above calculation should not be dispensed with.
Methane and gases having a considerable methane content have long been researched on to establish their physical properties and technical behavior.
Some of the properties, which have an effect on the combustion process in an engine, shall be explained hereunder:
- Ignitability of CH4 in a mixture with
air
CH4: 5 . . . 15 Vol %
air: 95 . . .85 Vol %
Mixtures which are leaner, i.e. CH4 content less than 5 Vol % or richer, i.e. CH4 content more than 15 Vol %, will not properly ignite with spark ignition.
-Combustion velocity cc in a mixture with air at a pressure of p = 1 bar
cc = 0.20 m/s at 7% CH4
cc =
0.38 m/s at 10% CH4
cc = 0.20 m/s at 13% CH4
The combustion velocity is a function of the volume percentage of the burnable component, here CH4. The highest value is near the stoichiometric air/fuel ratio, mostly at an excess air ratio of 0.8 . . . 0.9. It increases drastically at higher temperatures and pressures.
-Temperature at which CH4 ignites in a mixture with air
T1 = 918 K. . .1023K(=645°C...750°C)
- Compression ratio of an engine, e, at which temperatures reach values high enough for self-ignition in a mixture with air (CO2 content decreases ignitability, i.e. increases possible compression ratio)
e = 15...20
- Methane number, which is a standard value to specify a fuel's tendency to "knocking", i.e. uneven combustion and pressure development between TDC and BDC
Fig.4.4: "Knocking" in a p,
alfa-diagram of an engine
|
CH4, 100%: |
100 |
|
biogas (CH4 70%): |
130 |
|
for comparison: |
|
|
butane: |
10 |
|
propane: |
33.5 |
Methane and biogas are very stable against "knocking" and can therefore be used in engines of higher compression ratios than petrol engines. Fig. 4.4 illustrates the cause of the pressure and hence the force on the piston when the engine "knocks". Operation under such conditions will gradually destroy the engine.
- Stoichiometric air/fuel ratio on a mass basis at which the combustion of CH4 with air is complete but without unutilized excess air
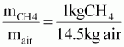
Biogases from different materials contain different percentages of hydrogen sulphide H2S, i.e. 0.10 . . . 0.50% Vol (1000 . . . 5000 ppm). As H2S is corrosive to metals especially in connection with water or humidity, its content should be as low as possible when used as a fuel in engines. Some engine manufacturers specify a maximum allowable value of 0.15% Vol; others allow more or give no data.
H2S can be removed by filtering with earth or with
iron oxide (e.g. filings) whereby the filters need to be regenerated or the
material exchanged periodically [24]. Recent experiments in a large biogas plant
in Ferkessedougou, Ivory Coast [25], have revealed that by purging a small
amount of air into the gas holder or store and allowing a reaction time of about
25 . . . 30 hours, a substantial
percentage, i.e. about 80%, of the
H2S is reduced to elementary sulphur which is deposited on surfaces
within the plant or on the floating scum. The amount of air allowed into the gas
holder/store needs however to be well dosed, preferably with a small dosage
pump. A mean value for the constant air supply is approx. 0.4 % Vol of the
constant gas production for a reduction of approx. 80% of the H2S,
e.g. from 0.5% Vol H2S to 0.1% Vol, which is adequate for engine
operation.
Depending on the type of biogas plant and piping, some indispensable solids can be drawn with the gas to the mixer. A simple filter in the form of a larger container filled with washed rubble or a tissue filter with no measurable pressure loss is recommendable in any system.
|
initial H2S content |
stoichiometric amount of oxygen as vol. % of biogas production |
stoichiometric amount of air as vol % of biogas production | |
|
in ppm |
in vol. % |
| |
|
500 |
0.05 |
0.025 |
0.125 |
|
1000 |
0.10 |
0.050 |
0.250 |
|
1500 |
0.15 |
0.075 |
0.375 |
|
2000 |
0.20 |
0.100 |
0.500 |
|
2500 |
0.25 |
0.125 |
0.625 |
|
3000 |
0.30 |
0.150 |
0.750 |
a) Stoichiometric amounts of oxygen or air to be added for an 85% reduction of the H2S content for different initial H2S content values.
b) H2S reduction from
initially 1500 ppm as a function of added
air.